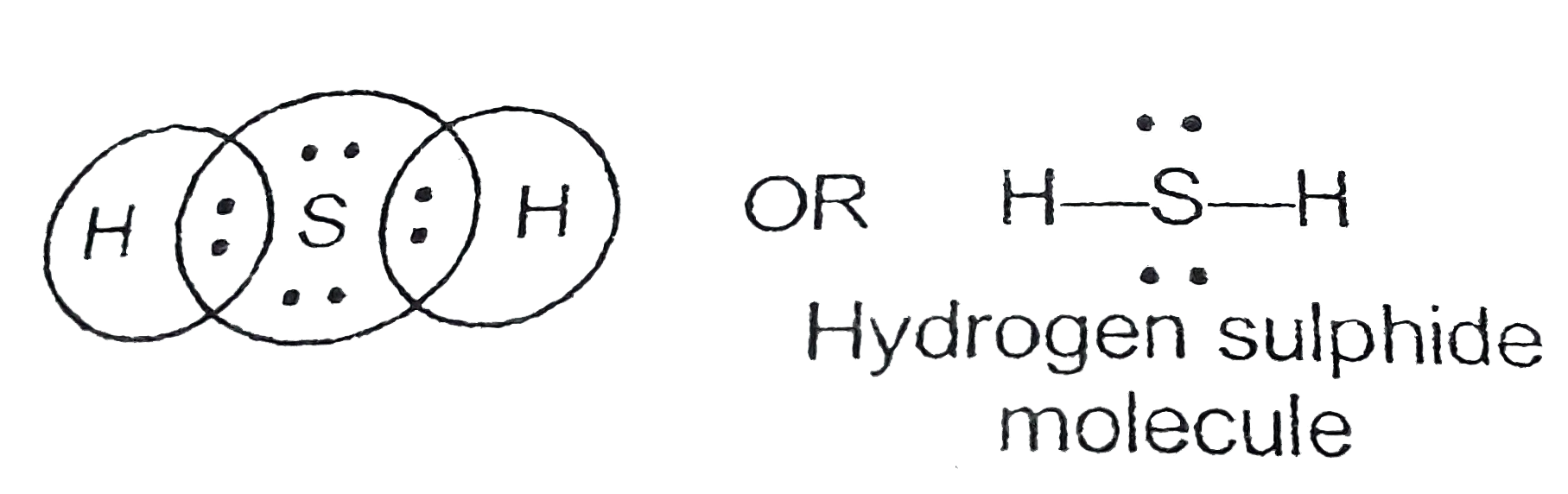
31+ H2S Lewis Structure Pictures Bepe Enthusiastic
Try the eBay way-getting what you want doesn't have to be a splurge. Browse A molecular! Find the deal you deserve on eBay. Discover discounts from sellers across the globe.

H2S Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar,Octet Rule
The first step is to sketch the molecular geometry of the H2S molecule, to calculate the lone pairs of the electron in the central sulfur atom; the second step is to calculate the H2S hybridization, and the third step is to give perfect notation for the H2S molecular geometry.
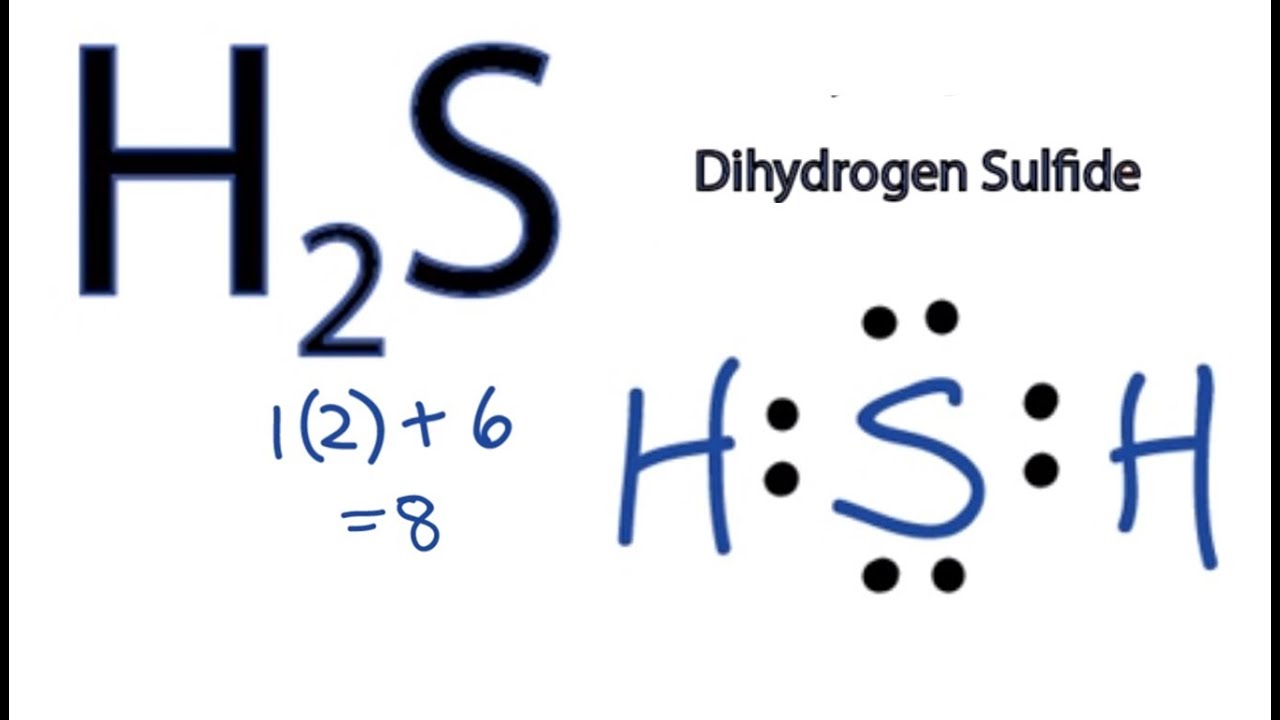
Estructura De Lewis H2s Estudiar
Step 1: Find out the total number of valence electrons in the molecule. Do take care of +, - signs while calculating. Step 2: Choose a central atom; generally the atom with the highest bonding sites. Step 3: Draw a skeletal structure with single bonds only. Step 4: Fill up the octet of the atoms with the remaining electrons.
Hydrogen Sulfide Molecule Photograph by Molekuul/science Photo Library Pixels
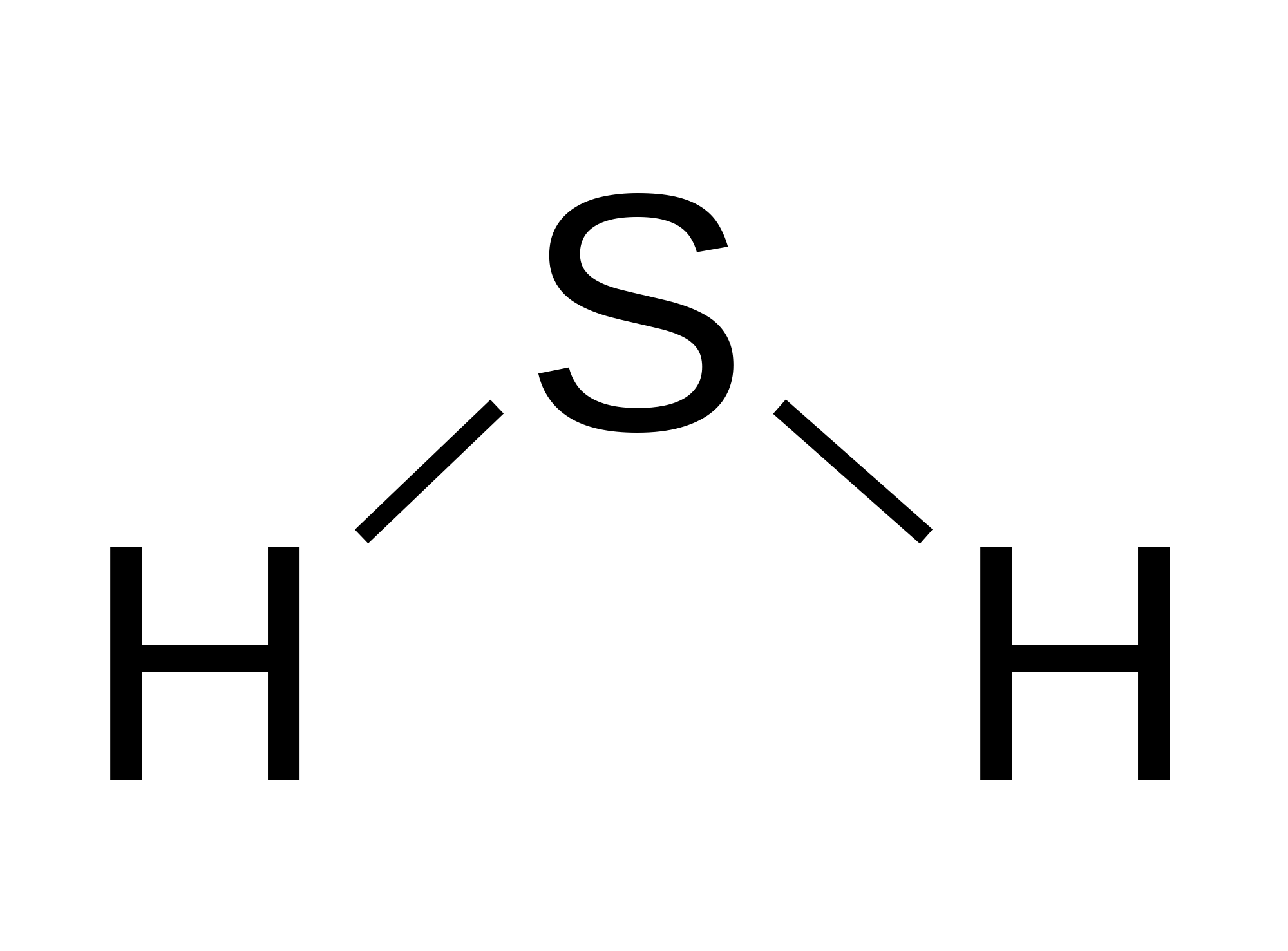
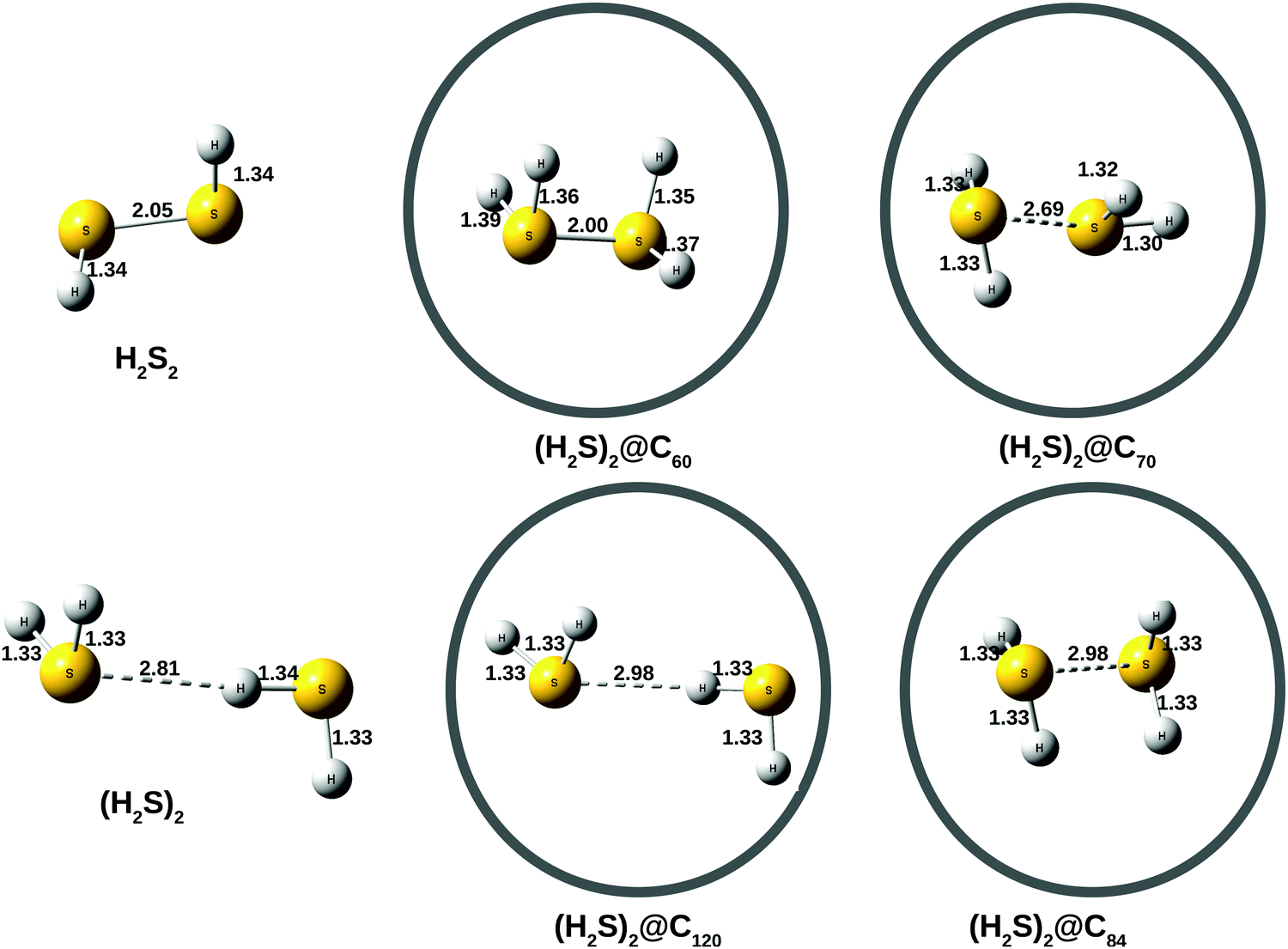
Molecular Geometry of H2S A. Determination of the shape of H2S molecule. By considering the arrangement of atoms and lone pairs around the central sulfur atom, one can determine the molecular geometry of H2S. H2S has two bonding pairs and two lone pairs of electrons on the sulfur atom. Due to the repulsion between lone pairs, the molecule.
H2S at emaze Presentation
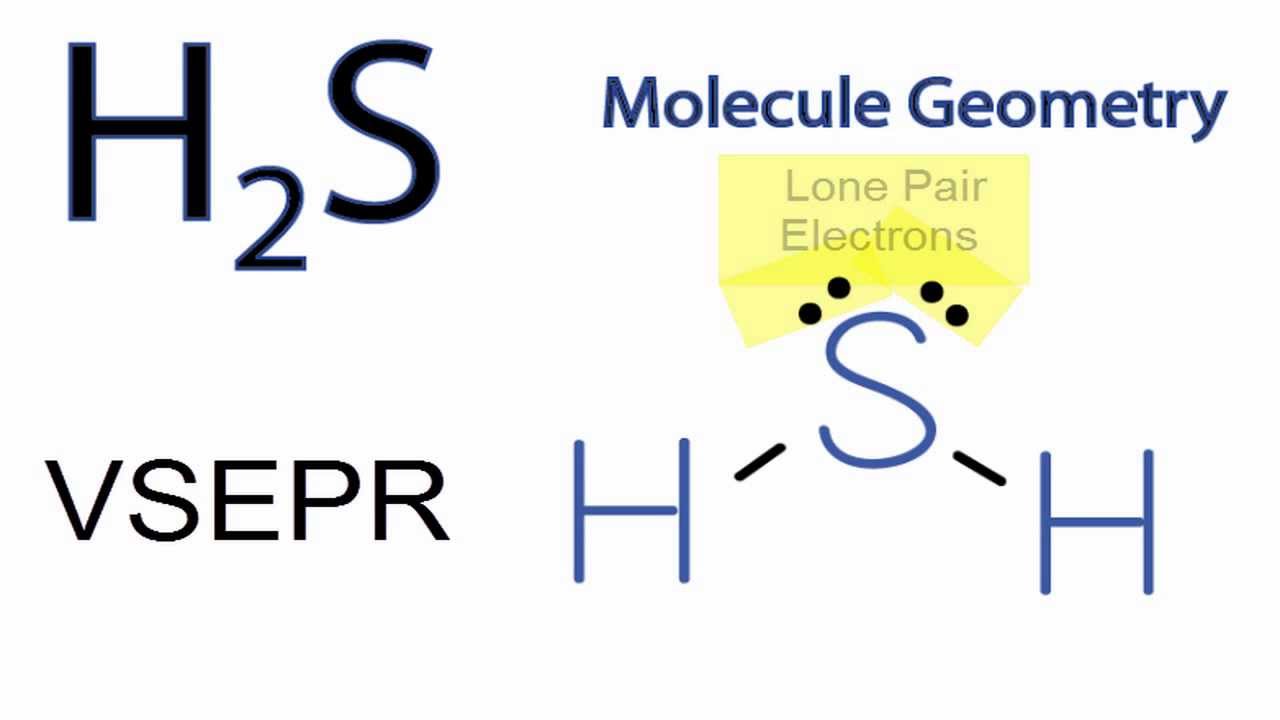
H2S Molecular Geometry / Shape and Bond Angles (Note: precise bond angle is 92.1 degrees.) Wayne Breslyn 715K subscribers Join Subscribe Subscribed 1 2 3 4 5 6 7 8 9 0 1 2 3 4 5 6 7 8 9 0 1 2 3 4.

H2s hydrogen sulfide molecule Royalty Free Vector Image
H2S is a chemical formula of Hydrogen Sulphide gas. It is highly toxic, poisonous and flammable. In this video, we will look at the molecular structure of an.

H2S Molecular Geometry Science Education and Tutorials
H2S Molecular and Electron Geometry based on the VSEPR theory, the steric number, Hybridization and expected bond angles.
H2s molecular geometry boothulsd
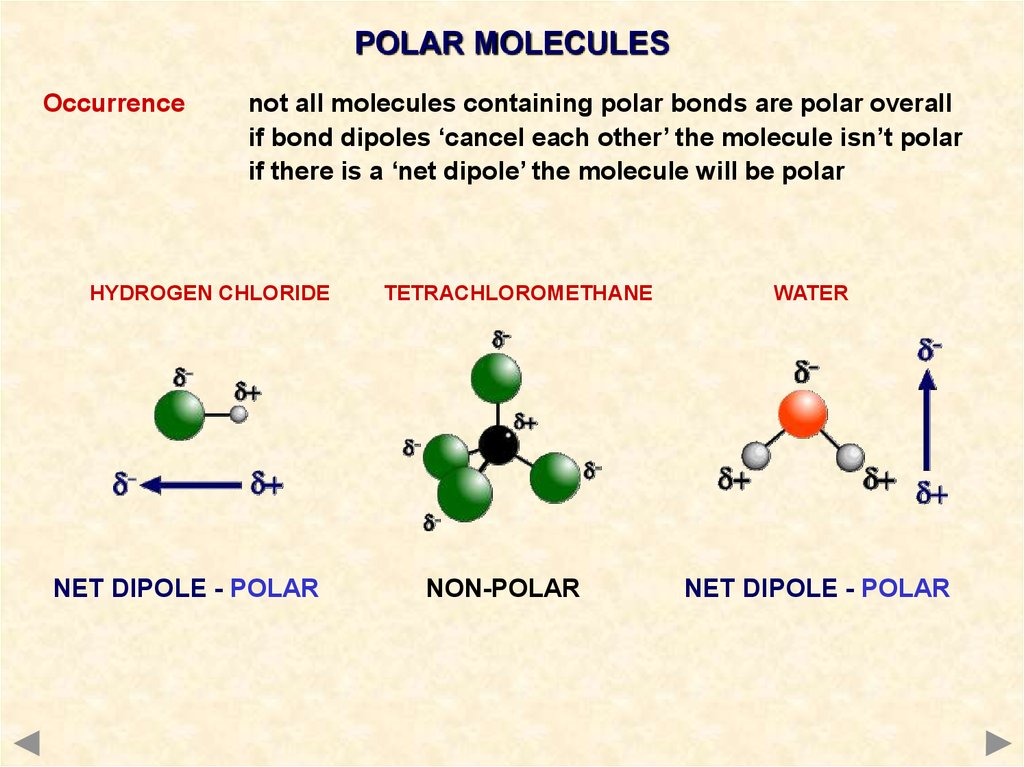
The total number of electrons around the central atom, S, is eight, which gives four electron pairs. Two of these electron pairs are bonding pairs and two are lone pairs, so the molecular geometry of \(\ce{H2S}\) is bent (Figure \(\PageIndex{6}\)). The bond dipoles cannot cancel one another, so the molecule has a net dipole moment.
H2S Molecular Geometry H2s Vsepr Term Top Hydrogen sulfide molecular structure
The hydrogen sulfide (H2S) molecule is classified as a polar molecule. The molecule of hydrogen sulfide (with tetrahedral or bent V-shaped molecular geometry) is tilted, the bond angles between sulfur and hydrogen are 92 degrees.

H2S Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram Techiescientist
What is CLF3 molecular geometry? 5. What is the hydrosulfuric acid formula? Related Links N2O Lewis Structure| Laughing Gas Step by Step Construction of H2s Lewis Structure The following are the steps to constructing the Lewis Structure: Step-1: Count the valence electrons of atoms.

H2S Lewis structure Polar, Molecular geometry, Science education
Written by Priyanka in Science Hydrogen Sulfide is a common chemical compound that is useful for analyzing inorganic compounds of metal ions. It has the chemical formula of H2S. The molecule has two Hydrogen atoms and a single Sulfur atom. H2S is also a precursor for elemental Sulfur.

H2S (Hydrogen sulfide) Molecular Geometry, Bond Angles YouTube
So, what are you waiting for? Dive into the article and learn all about hydrogen sulfide (H2S). Page Contents show How to draw lewis structure for H2S? The Lewis dot structure of hydrogen sulfide (H2S) consists of a sulfur (S) atom at the center. It is surrounded by 2 hydrogen (H) atoms, one on each side, by a single covalent bond.

Hydrogen sulfide H₂S Molecular Geometry Hybridization Molecular Weight Molecular Formula
An explanation of the molecular geometry for the H2S ion (Hydrogen sulfide) including a description of the H2S bond angles. The electron geometry for the Hyd.

H2s molecular geometry singleatila
¡Precios increíbles y alta calidad aquí en Temu. Envío gratuito en todos los pedidos. ¡Solo hoy, disfruta de todas las categorías hasta un 90% de descuento en tu compra.
H2S Molecular Geometry / Shape and Bond Angles YouTube
This video explains molecular geometry of H2S molecule by VSEPR theory. According to VSEPR theory, the shape of a covalent molecule depends upon the repulsion between the electron pairs in.
Understanding Hydrogen Sulfide (H2S) Aulick Chemical Solutions
The total number of electrons around the central atom, S, is eight, which gives four electron pairs. Two of these electron pairs are bonding pairs and two are lone pairs, so the molecular geometry of \(\ce{H2S}\) is bent (Figure \(\PageIndex{6}\)). The bond dipoles cannot cancel one another, so the molecule has a net dipole moment.